THE PERIODIC TABLE OF ELEMENTS
(updated)
This table is called periodic because all of the atoms found
in the same column (from 1 to 18), exhibit similar chemical properties.
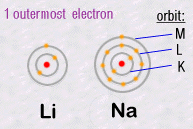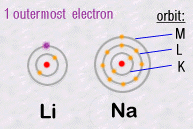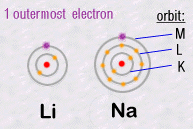
For example, in column 1, Lithium Li and Sodium Na have similar chemical
behaviours.
 Why?
Why?
Because the number of electrons in their outermost orbits are identical.
Li and Na each have a single electron circulating in their outermost orbits:
Thus it'll be this external electron which will be responsible for chemical
reactions with other atoms. Hence the same chemical properties and their
chemical grouping.
Atoms then are grouped in families:
|
true metals
|
|
transition metals
|
|
metalloids
|
|
non-metals
|
|
rare gases
|
|
lanthanides
|
|
lactinides
|
|
transuraniens = all of the elements derived from uranium
|
The family of rare gases exhibit a particular characteristic:
Helium, Neon, Argon etc are very stable atoms because they possess an external
layer of electrons which is complete. This layer completely full of electrons
is very stable and therefore the atom neither gives up nor accepts other
electrons.Chemical interaction between atoms are therefore nearly impossible
for the rare gases.
This unmasks a golden rule of chemistry: All
atoms tend to complete their external layer of electrons,
this by capturing or giving up electrons to other atoms.
The higher Z is, the larger is the nucleus and
the more it becomes unstable: it then has a tendancy to fragment into other
atoms which are smaller and more stable: such atoms are radioactive
atoms.
In the periodic table, the radioactive atoms are:
-
Technetium 43
-
Samarium 62
-
all elements starting from Polonium where Z is greater
than or equal to 84.


 Why?
Why?