THE CLASSIC ATOM 2
or
The neutron makes the isotope
or
The neutron makes the isotope
![]() The
neutron and isotopes
The
neutron and isotopes
The neutron is very similar to the proton: of a nearly equivalent mass,
but electrically neutral.
| The neutron and the proton form the family of nucleons. |
For a given type of atom, the number of neutrons (designated N)
can vary, but it is generally fairly close to the number of protons Z,
except for in very heavy atoms.
| Number of protons | Number of neutrons | isotopes |
| Z = 92 | N = 143 | Uranium U235 |
| Z = 92 | N = 146 | Uranium U238 |
For example, natural Uranium exists in two forms: its nucleus can have
92 protons + 143 or 146 neutrons.
We call these two varieties of Uranium isotopes of Uranium,
designated U 235 (because 92 protons + 143 neutrons = 235 nucleons) and
U 238.
With U 235, we call 235 the atomic mass of the atom, designated A.
|
|
Another example, if Hydrogen (in addition to its single proton) possessed
one or two neutrons, it would become an isotope of hydrogen (in this case
baptised deuterium or tritium).
| Number of protons | Number of neutrons | isotopes |
| Z = 1 | N = 0 | Normal Hydrogen |
| Z = 1 | N = 1 | Heavy Hydrogen
= Deuterium |
| Z = 1 | N = 2 | Ultra heavy Hydrogen
= Tritium |
Watch this animated example for Carbon 12 and its isotope Carbon 13:
![]()
Note that if you add to C, not a neutron, but a proton, you change its nature and it becomes another atom (here Carbon becomes Nitrogen N):
![]()
![]()
It is worth noting that certain atomic isotopes such as Carbon 14 or Potassium 40 are radioactive and are therefore unstable. Radioactive atoms are also called radioelements.
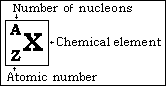
Notation:
An atom like Carbon is written symbolically as ![]() with A=12 and Z=6.
with A=12 and Z=6.
Its isotope, Carbon 14, is therefore written ![]() .
.