or
electrons, unifiers of atoms
![]() Electrons
and chemistry
Electrons
and chemistry
The electron is denoted in physics with an e-.
Normally, an atom has as many electrons (of - charge) as protons (of + charge). The atom is therefore overall neutral in terms of its electrical charge.
In the same way, a Carbon atom C can lose two electrons and become a cation with two surplus positive charges, this would be denoted by C2-. In contrast, if it gained an additional electron, it would become an anion C+
![]()
![]()
![]() Chemical
bonds
Chemical
bonds
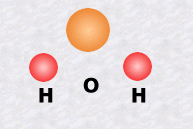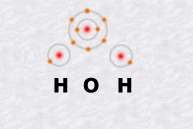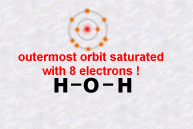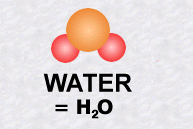
Electrons are organised in successive layers around the nucleus. These layers are filled according to very strict rules defined by a quantum law which will be revealed later on (Pauli's law of exclusion).
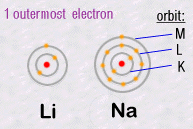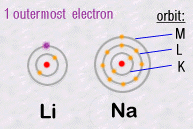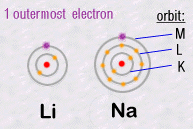 The important
thing to remember is that it is the outer most electrons which are responsible
for all of the chemistry, because it's them that can join together two
separate atoms creating chemical bonds. For example, two atoms can each
share one of their electrons and then create what we call a covalent
chemical bond. As a result of such bonds, atoms can assemble themselves
into an infinite diversity and complexity of molecules.
The important
thing to remember is that it is the outer most electrons which are responsible
for all of the chemistry, because it's them that can join together two
separate atoms creating chemical bonds. For example, two atoms can each
share one of their electrons and then create what we call a covalent
chemical bond. As a result of such bonds, atoms can assemble themselves
into an infinite diversity and complexity of molecules.
![]()
![]() Molecules
and macromolecules
Molecules
and macromolecules
 Molecules
are the assemblies which are at the root of both inert and living matter.
Molecules
are the assemblies which are at the root of both inert and living matter.
When the number of molecules in an atom gets up to several hundred,
we begin to speak of macromolecules. Certain polymers can be made
up of several millions of atoms!
In biochemistry (or organic chemistry), molecules such as proteins,
lipids, glucides and nucleic acids (constituants of DNA, which stores the
genetic code) organise themselves into superstructures (cellular components);
these are the constituants of the living cell, the basic building block
of all living organisms.
The water molecule, one of the simplest, is made up of one atom of oxygen and two atoms of hydrogen.
![]()
![]() How
can we imagine the classical atom?
How
can we imagine the classical atom?
Imagine that the nucleus of hydrogen (a single proton) measured 1 millimeter: it would then be millions of times bigger than it is in reality (enlarged by 1,000,000,000,000 x) !
The volume of the nucleus is a million milliard times smaller than that
of the atom;
the volume of the atom is thus made up of at least 99,9999999999999
% empty vacuum!
(source: David Calvet of CNRS
Marseille, labo in2p3 a reference web site on nuclear physics, which
is extremely clear (-- translators note -- clear in french that is).
![]() Can
we "see" an atom?
Can
we "see" an atom?
Since very recently and indirectly yes, and with the aid of two ultra-powerfull microscopes:


![]()
|
|
|