or
Orbitals and quantum indeterminacy
![]() The
quantum atom
The
quantum atom
If we return to our atom; quantum physics is going to render Bohr's model redundant and replace it by a model more vague... more difficult to represent.
 In
effect, we've seen that the quantum electron is a double agent, victim
of wave-particle duality. The wave associated with the electron corresponds
in fact to a probability to find the said quantum electron at a given location.
In
effect, we've seen that the quantum electron is a double agent, victim
of wave-particle duality. The wave associated with the electron corresponds
in fact to a probability to find the said quantum electron at a given location.
The particle is no longer a classical material point but a bundle of
probabalistic waves, a superimposition of potential movements. Electronic
orbits must give way to the notion of orbitals, (a sort of fuzzy
and probabalistic sphere), in which the electron will be in some ways diluted
all around the nucleus.
It's not until physicists interact with the atom to observe the electron,
that it appears as a particle: It's as though the undulatory electronic
cloud suddenly reduces into a solid material particle. If it is necessary
to risk an image to illustrate this curious phenomena, one could imagine
the electron as a sub-marine which emerges, long enough for a measurement,
from its probabalistic ocean. Later, it submerges and it will be impossible
to an observer from the surface to localise it with any precision: one
could do no more that define the volume of the ocean where the submarine
could probably be found.
| electronic orbital | observed electron |
| wave like nature | particle like nature |
 |
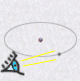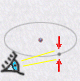 |
This new model of electronic clouds obeys a key principle of quantum
physics: The Heisenbergs indeterminacy principle.
If we were reduced to the scale of the atom, what would we see?
|
|
|
![]()
![]() Heisenbergs
principle of indeterminacy
Heisenbergs
principle of indeterminacy
 Besides
the fact that the german physicist Werner Heisenberg is the father
of this law (sometimes also called the uncertainty principle), what
does this principle tell us? that it is impossible to determine with precision
and simultaneously, the position and the velocity of a particle like the
electron. The notion of an exact trajectory doesn't make any sense for
particles. This quantum paradox (another one!) is associated with the difficulty
to observe an electron... How do you observe it?
Besides
the fact that the german physicist Werner Heisenberg is the father
of this law (sometimes also called the uncertainty principle), what
does this principle tell us? that it is impossible to determine with precision
and simultaneously, the position and the velocity of a particle like the
electron. The notion of an exact trajectory doesn't make any sense for
particles. This quantum paradox (another one!) is associated with the difficulty
to observe an electron... How do you observe it?
We can't observe something without illuminating it with light. Now at
the scale of the infinitesimaly small, that poses a completely new problem.
The least photon which strikes or interacts with an electron will modify
its initial trajectory or make it change orbitals. At this scale, the photon
becomes a projectile which could determine the position of the electron,
but which would at the same time modify its speed and its trajectory; these
cannot therefore be known at the same time. The slightest measurement interferes
with the object being measured, and changes it!
![]() the
quantum owlet
the
quantum owlet
Dare we have a new image to illustrate this principle:
Deep in the woods at night, a nature lover hears the hooting of an
owl. If he would like, at the same time, see the feathered creature, he
would have to turn a torch on him: But then it's a good bet that the surprised
owl will stop singing. This gives rise to the insoluble dilema: We can't
both hear and see the owl at the same time... Alas!

![]()