 RADIOACTIVITY
RADIOACTIVITY
![]() Discoveries
Discoveries
 One
day in 1896, Henri Becquerel (by chance according to legend?) arranged
in his cupboard, a packet of uranium salt beside an unexposed photographic
plate. Several days later, he took out the plate and developed it.
To his surprise, he noticed that the photographic plate had been exposed
without having been exposed to the light. Having repeated this experiment,
he concluded that Uranium spontaneously emits what he called "uranic rays".
One
day in 1896, Henri Becquerel (by chance according to legend?) arranged
in his cupboard, a packet of uranium salt beside an unexposed photographic
plate. Several days later, he took out the plate and developed it.
To his surprise, he noticed that the photographic plate had been exposed
without having been exposed to the light. Having repeated this experiment,
he concluded that Uranium spontaneously emits what he called "uranic rays".
 In
1898, Marie Curie discovered that pitchblend, a uranium ore, emits
more radiation than uranium itself. She deduced that this ore contains,
in very small quantities, one or more elements much more active that uranium.
With the assistance of her husband Pierre Curie and after two years of
effort, she arrived at isolating two new elements: Polonium (named thus
in tribute to her homeland) and Radium. It was then that Marie Curie invented
the word "radioactivity".
In
1898, Marie Curie discovered that pitchblend, a uranium ore, emits
more radiation than uranium itself. She deduced that this ore contains,
in very small quantities, one or more elements much more active that uranium.
With the assistance of her husband Pierre Curie and after two years of
effort, she arrived at isolating two new elements: Polonium (named thus
in tribute to her homeland) and Radium. It was then that Marie Curie invented
the word "radioactivity".
![]()
![]() The
3 radiations
The
3 radiations
There exist three varieties of radioactivity characterised by the emission of different rays emitted by the nucleus of the atom:
|
These three varieties of radioactivity are not emitted simultaneously. Each nuclear reaction of an atom emits only one single type of radiation at a time!
For example, radioactive Uranium-238 emits an alpha ray
and thus loses 4 nucleons (2 protons + 2 neutrons): U 238 thereby transforms
itself into Thorium-234 (because 2 protons less - that changes an
atom!).
![]()
![]() Transmutation
and half-life
Transmutation
and half-life
Radioactivity is a spontaneous transmutation of one atom into another atom with the emission of radiation.
These transmutations, ancient dreams of the alchemists of the middle
ages, take place more or less rapidly, depending upon a characteristic
interval of a radio-element which is called its radioactive half-life.
Let's imagine an extremely large population of radioactive atoms, all
identical: the half-life of this population is equal, by definition, to
the length of time after which half of the atoms which composed the population
at the start have transmuted into other elements; after a second period
of time, the remaining population is once again divided by two and therefore
makes a quarter of the initial number, and so on.
This half-life can vary, depending upon the radio-element, from a few
fractions of a second to several milliard years. Tellurium 128
has a half-life of 1.5 x 1024 years, that
is to say one hundred thousand milliard, milliard times the age of the
Universe... (one hundred thousand billion, billion in american english)
![]()
![]() Natural
or artificial?
Natural
or artificial?
Radioactivity has not been invented by man. It has existed since the
beginning of the universe: We speak of natural radioactivity when
it is due to the durable radio-elements formed in the stars which have
not yet found their stable state: they will end up transforming themselves
into stable atoms. This radioactivity is very important and releases a
very large amount of energy which without doubt maintains the magma in
a molten state beneath the earths crust.
For example uranium, thorium, radium and the isotopes
carbon
14, radon 222 and potassium 40 are natural radioactive
elements present in the minerals of the earth, in thermal waters and in
the air.

We speak of articial radioactivity when referring to elements
fabricated by man. In this case, these atoms are very heavy (with
a high atomic number Z), very unstable and therefore have a very short
half-life. Physicists create these artificial radio-elements by bombarding
natural atoms whith protons or alpha particle: the nuclei of these atoms
acquire additional protons which transform them into new heavier atoms.
Radioactivity is produced frequently by large unstable nuclei....
This transformation of atomic nuclei is called a nuclear reaction of
which much talk is heard about two types: nuclear fission and nuclear
fusion.
![]()
![]() Nuclear
fission
Nuclear
fission
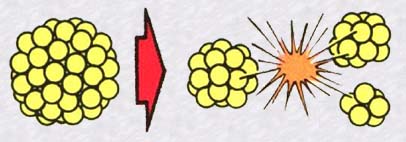
Whenever the nucleus of a heavy atom (like uranium 235) fissions (fragments) into two smaller nuclei, it produces a remarkable event: the sum of the masses of these two remaining nuclei is less than the mass of the original large nucleus. Where has the missing mass gone? It has transformed itself into pure energy (Einsteins mass-energy equivalence), an enormous quantity of energy. Furthermore, in the case of uranium 235, the fission of the nucleus can be provoked by a single neutron and, a very important detail, this fission in turn produces other neutrons which can themselves break apart other uranium nuclei ...We are about to witness a chain reaction.
To see a short explanetary
film ( .avi format of 991 KBytes) on the chain reaction, click on the atomic
explosion below:
![]()
![]() Nuclear
fusion
Nuclear
fusion
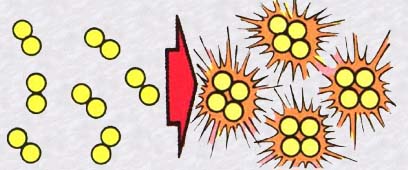
In broad terms it's the inverse of fission.
Two light atomic nuclei (like hydrogen) crash into each other and fuse
together into a single bigger nucleus. Now the final mass of this big nucleus
is smaller than the sum of the masses of the two initial nuclei, which
is where we get an enormous release of energy produced by the annihilation
of this difference of mass.
In order to be able to provoke such a fusion reaction, it is necessary
to force the nuclei, all positively charged, to move together and to overcome
their mutual repulsion (like two lovers who repulse each other): This is
not possible except at very high temperatures (the temperature corresponding
to the intensity necessary to get the particles to crash into each other).
This is why the nuclear fusion reaction is also called a thermonuclear
reaction (thermo = heat).
H BOMB (H for Hydrogen) =
This uncontrolled reaction is used in the hydrogen bomb or H bomb.
This reaction is also seen in the heart of our Sun where temperatures
reach hundreds of millions of degrees. Nuclear fusion there is auto-regulated
by an equilibrium between the pressure of the explosion produced and the
force of gravity which crushes the enormous mass of the Sun in on itself.
This reaction is the source of the energy which gives rise to life on Earth.
![]()
|
|
Radio Active with DJ Atomic emits 3 types
of program:
|